Data Submission Process Guide
Create an ImmPort Account

In order to submit data to ImmPort, you will need to be logged into your ImmPort account. If you do not have an account, you can easily register with your email address here: ImmPort Registration
Access a Workspace

A workspace is a private, access controlled environment for uploading data. The data in the workspace is only visible to those granted access to it, and the data will remain in the workspace after it is made public in an ImmPort Study.
If you are a new member to ImmPort, complete this Workspace Request Form to request the ImmPort create a workspace for you.
If you are a new member, but your team has previously loaded data to ImmPort, the ImmPort team can add you to the workspace your team has access to. Please have a team member who currently has workspace access complete this Workspace Request Form and request to add you to a workspace.
If you are an existing member and you have an existing workspace, you can add a new study to that workspace. If you plan to use the same subjects accross multiple studies in ImmPort, please upload the study data in the same workspace.
Please contact the ImmPort team with any additional questions: ImmPort_Helpdesk@immport.org
Register a New Study

Once you have access to a workspace, the first step is to register a new study. “Registering” a study refers to populating metadata needed to describe the basic design of the study.
There are two options to fill out the required details and register a study:
Option 1) Study Registration Wizard (Recommended)
Use the online Study Registration Wizard (SRW) form to register your study. As you fill out the form, the SRW ensures that the metadata is properly formatted. Once you fill out all pages and submit the SRW form, your study will be registered. A tutorial of the Study Registration Wizard can be found here: SRW Documentation
Register your study with the SRW here: Study Registration Wizard
Option 2) Basic Study Design Template
Download an excel version of the “Basic Study Design” and "Protocol" submission templates, populate the contents, and upload the submission templates to ImmPort. Before populating, validating, and uploading these submission templates, please read the "How to Complete Submission Templates” Section in the documentation below. Additional documentation on the Basic Study Design template and Protocols template can be found here:
How do I know if I should use the SRW or Basic Study Design Template? Both the SRW and Template will register a study in the database, and will capture the basic metadata associated with the study. The SRW is typically a faster process, and is recommended if you do not want to parse any additional data into the ImmPort database, other than the metadata captured in the SRW. If this is the case, please upload any supporting data as a native format "Study File" during the final phase of registering a study with the SRW. If your data does not contain any human or animal subjects, we recommend this Study File approach.
If you do plan to parse additional data, you can use either the SRW or Basic Study Design Template to capture the basic study metadata. To add additional data, please review the next section on completing, Validating, and Uploading Data.
Complete, Validate, and Upload Data

Once you have registered your study either via the SRW or via submission templates, you will use additional templates to upload data into the newly registered study.
Submission Template Upload Order
Many templates reference information from other templates. Therefore, there is a set order to upload the templates. In general, reading the ImmPort Data Model left to right can help indicate the order of template uploads. If one submission template requires another one to be uploaded first, you should either upload the submission templates separately in the correct order OR include them within the same upload.
Please see the below decision flow diagram on the order of submission templates to complete and upload for a typical study. If you study content does not fit into the common upload structure depicted by the flow diagram, please contact the ImmPort Helpdesk for help supporting your study: ImmPort_Helpdesk@immport.org
Typical ImmPort Submission Template Upload Order:
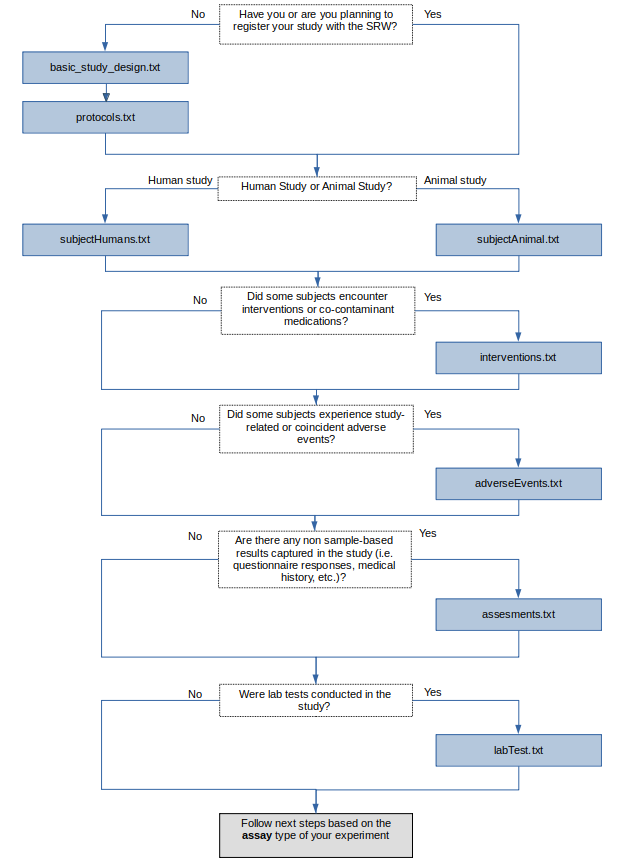
ImmPort supports results data for multiples assay types, each of which require different templates to be uploaded. Once the above flow diagram has been followed, please follow one of the flow diagrams in the documents linked based on your experiment’s assay type:
- CyTOF
- ELISA
- Elispot
- Flow Cytometry
- Gene Expression
- RNA Sequencing
- Genotyping
- Hemagglutination Inhibition Assays
- HLA Typing
- Image Histology
- KIR Assays
- Metabolomics Mass Spectrometry
- Multiplex Bead Array Assay
- Neutralizing Antibody Titer
- Proteomics Mass Spectrometry
- QRT-PCR
- Other
How to Complete Submission Templates
The steps below provide general guidance on how to populate the submission templates. For each submission template, please complete the following steps:
-
Download the submission template of interest: Submission Templates. It is recommended to download the Excel version to complete the submission template, however the file must be saved and uploaded as a tabular .txt file.
-
Fill out the content of the submission template in excel. For additional help with completing the submission templates, please see this document: Excel Submission Template Tutorial
-
Save the completed submission template as a tabular .txt file.
-
Recommended: Log into your ImmPort account and validate the submission template: Validate Templates. This step completes a test upload of the template to check for errors. Within 5 minutes of validation upload, feedback will be provided to the user on the ImmPort site, as well as by email, on whether the upload was successful, and if not, what errors occurred. For help with trouble-shooting common validation errors, please see: Common Validation Errors or contact the ImmPort team: ImmPort_Helpdesk@immport.org
-
Log into your ImmPort account and upload the submission template here: Upload Templates. As with validation, feedback will be provided to the user via email and on the ImmPort site on the success of the upload. Multiple submission templates can be uploaded at once in the same upload package. Ensure the uploads are in the correct order. At most, 512 MB can be uploaded at once. To upload files larger than 512MB, please upload via Aspera: IBM Aspera Connect
Note: If you need to make changes to information within a submission template after uploading, please contact the ImmPort team at ImmPort_Helpdesk@immport.org. The basic_study_design.txt template is an exception; study_design_edit.txt template can update the content of the study design. Please read the instructions on this submission template here: Study Design Edit Template
Additional Documentation:
-
For details on how to complete specific submission templates, go to Template Documentation: Introduction and then select your submission template of interest under the "Templates" dropdown on the documentation navigation menu.
-
Full, detailed documentation of all submission templates can be found here: ImmPort Upload Template Description
-
Sample Data Upload Packages can be found here: Example Data Packages
Share Study in Data Release

Contact the ImmPort team at ImmPort_Helpdesk@immport.org to let them know you are ready to share your study.
Once you contact ImmPort indicating you are ready to share, the ImmPort team will conduct a quality check of the data, provide feedback, and will discuss with you when you would like the data shared. ImmPort will then share your study in a data release. Currently, ImmPort has a monthly data release schedule. Please contact the ImmPort team at ImmPort_Helpdesk@immport.org for any additional questions on data releases. The current data release schedule can be viewed here.